Hjelp oss med å gjøre åpenhet om mat til det normale!
Som en ideell organisasjon, er vi avhengige av dine donasjoner for å fortsette å informere forbrukere verden over om hva de spiser.
Matrevolusjonen starter med deg!
Melkesjokoladekjeks - Freia - 184g
Melkesjokoladekjeks - Freia - 184g
Denne produktsiden er ikke fullstendig. Du kan hjelpe med å ferdigstille den ved å redigere den og legge til mere data fra bildene vi har, eller ved å ta flere bilder i appen for Android eller iPhone/iPad. Takk!
×
Strekkode: 7622300591052 (EAN / EAN-13)
Mengde: 184g
Merker: Freia, Mondelez International, Mondelez
Kategorier: en:Snacks, en:Sweet snacks, en:Biscuits and cakes, Kjeks, en:Chocolate biscuits, en:Drop cookies, en:Chocolate chip cookies
Etiketter, sertifiseringer, priser:
Grønt Punkt, en:Rainforest Alliance

Samsvarer med dine preferanser
Helse
Ingredienser
-
19 ingredienser
Hvetemel, melkesjokolade 22,5 % (sukker, tørrmelk, kakaosmør, kakaomasse, emulgator (soyalecitin), aroma), palmeolje, sukker, hevemidler (ammoniumhydrogenkarbonat, natriumhydrogenkarbonat, dinatriumdifosfat), salt, aroma, fargestoff (betakaroten). Kan inneholde nøtter, egg.Allergener: Gluten, Melk, SoyaSpor: Egg, Nøtter
Matprosessering
-
Ultrabearbeidede matvarer
Elementer som indikerer at produkter er i en:4 - Ultra processed food and drink products-gruppen:
- Tilsetningsstoff: E160a - Karotener
- Ingrediens: Farge
- Ingrediens: Emulgeringsmiddel
- Ingrediens: Aroma
Matvarer er inndelt i 4 grupper i henhold til bearbeidingsgraden:
- Ubearbeidet eller minimalt bearbeidet mat
- Bearbeidede kulinariske ingredienser
- Bearbeidet mat
- Ultrabearbeidede matvarer
Bestemmelsen av gruppa er basert på kategorien til produktet og på ingrediensene den inneholder.
Tilsetningsstoffer
-
E160a - Karotener
Carotene: The term carotene -also carotin, from the Latin carota, "carrot"- is used for many related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but in general cannot be made by animals -with the exception of some aphids and spider mites which acquired the synthesizing genes from fungi-. Carotenes are photosynthetic pigments important for photosynthesis. Carotenes contain no oxygen atoms. They absorb ultraviolet, violet, and blue light and scatter orange or red light, and -in low concentrations- yellow light. Carotenes are responsible for the orange colour of the carrot, for which this class of chemicals is named, and for the colours of many other fruits, vegetables and fungi -for example, sweet potatoes, chanterelle and orange cantaloupe melon-. Carotenes are also responsible for the orange -but not all of the yellow- colours in dry foliage. They also -in lower concentrations- impart the yellow coloration to milk-fat and butter. Omnivorous animal species which are relatively poor converters of coloured dietary carotenoids to colourless retinoids have yellowed-coloured body fat, as a result of the carotenoid retention from the vegetable portion of their diet. The typical yellow-coloured fat of humans and chickens is a result of fat storage of carotenes from their diets. Carotenes contribute to photosynthesis by transmitting the light energy they absorb to chlorophyll. They also protect plant tissues by helping to absorb the energy from singlet oxygen, an excited form of the oxygen molecule O2 which is formed during photosynthesis. β-Carotene is composed of two retinyl groups, and is broken down in the mucosa of the human small intestine by β-carotene 15‚15'-monooxygenase to retinal, a form of vitamin A. β-Carotene can be stored in the liver and body fat and converted to retinal as needed, thus making it a form of vitamin A for humans and some other mammals. The carotenes α-carotene and γ-carotene, due to their single retinyl group -β-ionone ring-, also have some vitamin A activity -though less than β-carotene-, as does the xanthophyll carotenoid β-cryptoxanthin. All other carotenoids, including lycopene, have no beta-ring and thus no vitamin A activity -although they may have antioxidant activity and thus biological activity in other ways-. Animal species differ greatly in their ability to convert retinyl -beta-ionone- containing carotenoids to retinals. Carnivores in general are poor converters of dietary ionone-containing carotenoids. Pure carnivores such as ferrets lack β-carotene 15‚15'-monooxygenase and cannot convert any carotenoids to retinals at all -resulting in carotenes not being a form of vitamin A for this species-; while cats can convert a trace of β-carotene to retinol, although the amount is totally insufficient for meeting their daily retinol needs.Kilde: Wikipedia (Engelsk)
-
E160ai - Betakaroten
Beta-Carotene: β-Carotene is an organic, strongly colored red-orange pigment abundant in plants and fruits. It is a member of the carotenes, which are terpenoids -isoprenoids-, synthesized biochemically from eight isoprene units and thus having 40 carbons. Among the carotenes, β-carotene is distinguished by having beta-rings at both ends of the molecule. β-Carotene is biosynthesized from geranylgeranyl pyrophosphate.β-Carotene is the most common form of carotene in plants. When used as a food coloring, it has the E number E160a. The structure was deduced by Karrer et al. in 1930. In nature, β-carotene is a precursor -inactive form- to vitamin A via the action of beta-carotene 15‚15'-monooxygenase.Isolation of β-carotene from fruits abundant in carotenoids is commonly done using column chromatography. It can also be extracted from the beta-carotene rich algae, Dunaliella salina. The separation of β-carotene from the mixture of other carotenoids is based on the polarity of a compound. β-Carotene is a non-polar compound, so it is separated with a non-polar solvent such as hexane. Being highly conjugated, it is deeply colored, and as a hydrocarbon lacking functional groups, it is very lipophilic.Kilde: Wikipedia (Engelsk)
-
E500 - Natriumkarbonater
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Kilde: Wikipedia (Engelsk)
-
E500ii - Natriumbikarbonat
Sodium carbonate: Sodium carbonate, Na2CO3, -also known as washing soda, soda ash and soda crystals, and in the monohydrate form as crystal carbonate- is the water-soluble sodium salt of carbonic acid. It most commonly occurs as a crystalline decahydrate, which readily effloresces to form a white powder, the monohydrate. Pure sodium carbonate is a white, odorless powder that is hygroscopic -absorbs moisture from the air-. It has a strongly alkaline taste, and forms a moderately basic solution in water. Sodium carbonate is well known domestically for its everyday use as a water softener. Historically it was extracted from the ashes of plants growing in sodium-rich soils, such as vegetation from the Middle East, kelp from Scotland and seaweed from Spain. Because the ashes of these sodium-rich plants were noticeably different from ashes of timber -used to create potash-, they became known as "soda ash". It is synthetically produced in large quantities from salt -sodium chloride- and limestone by a method known as the Solvay process. The manufacture of glass is one of the most important uses of sodium carbonate. Sodium carbonate acts as a flux for silica, lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass produced insoluble. This type of glass is known as soda lime glass: "soda" for the sodium carbonate and "lime" for the calcium carbonate. Soda lime glass has been the most common form of glass for centuries. Sodium carbonate is also used as a relatively strong base in various settings. For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It acts as an alkali because when dissolved in water, it dissociates into the weak acid: carbonic acid and the strong alkali: sodium hydroxide. This gives sodium carbonate in solution the ability to attack metals such as aluminium with the release of hydrogen gas.It is a common additive in swimming pools used to raise the pH which can be lowered by chlorine tablets and other additives which contain acids. In cooking, it is sometimes used in place of sodium hydroxide for lyeing, especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning. In taxidermy, sodium carbonate added to boiling water will remove flesh from the bones of animal carcasses for trophy mounting or educational display. In chemistry, it is often used as an electrolyte. Electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately.Kilde: Wikipedia (Engelsk)
-
E503
Ammonium carbonate: Ammonium carbonate is a salt with the chemical formula -NH4-2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.Kilde: Wikipedia (Engelsk)
-
E503ii - Ammoniumhydrogenkarbonat
Ammonium carbonate: Ammonium carbonate is a salt with the chemical formula -NH4-2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.Kilde: Wikipedia (Engelsk)
Analyse av ingredienser:
-
en:Palm oil
Ingredienser som inneholder palmeolje: Palmeolje
-
en:Non-vegan
Ikke-veganske ingredienser: Melkesjokolade, MelkepulverNoen ingredienser kunne ikke gjenkjennes.
Vi trenger din hjelp!
Du kan hjelpe oss med å gjenkjenne flere ingredienser og bedre analysere ingredienslista for dette produktet og andre ved å:
- Rediger denne produktsiden for å korrigere skrivefeil i ingredienslista, og/eller for å fjerne ingredienser på andre språk og setninger som ikke er knyttet til ingrediensene.
- Legg inn nye oppføringer, synonymer eller oversettelser til våre flerspråklige ingredienslister, ingrediensbearbeidingsmetoder, og etiketter.
Bli med i kanalen #ingredients på vårt Slack-samtalested og/eller lære om ingrediensanalyse på wikien vår, hvis du ønsker å hjelpe til. Tusen takk!
-
en:Vegetarian status unknown
Ugjenkjente ingredienser: DinatriumdifosfatNoen ingredienser kunne ikke gjenkjennes.
Vi trenger din hjelp!
Du kan hjelpe oss med å gjenkjenne flere ingredienser og bedre analysere ingredienslista for dette produktet og andre ved å:
- Rediger denne produktsiden for å korrigere skrivefeil i ingredienslista, og/eller for å fjerne ingredienser på andre språk og setninger som ikke er knyttet til ingrediensene.
- Legg inn nye oppføringer, synonymer eller oversettelser til våre flerspråklige ingredienslister, ingrediensbearbeidingsmetoder, og etiketter.
Bli med i kanalen #ingredients på vårt Slack-samtalested og/eller lære om ingrediensanalyse på wikien vår, hvis du ønsker å hjelpe til. Tusen takk!
-
Detaljer fra analysen av ingrediensene
Vi trenger din hjelp!
Noen ingredienser kunne ikke gjenkjennes.
Vi trenger din hjelp!
Du kan hjelpe oss med å gjenkjenne flere ingredienser og bedre analysere ingredienslista for dette produktet og andre ved å:
- Rediger denne produktsiden for å korrigere skrivefeil i ingredienslista, og/eller for å fjerne ingredienser på andre språk og setninger som ikke er knyttet til ingrediensene.
- Legg inn nye oppføringer, synonymer eller oversettelser til våre flerspråklige ingredienslister, ingrediensbearbeidingsmetoder, og etiketter.
Bli med i kanalen #ingredients på vårt Slack-samtalested og/eller lære om ingrediensanalyse på wikien vår, hvis du ønsker å hjelpe til. Tusen takk!
: _Hvetemel_, melkesjokolade 22.5% (sukker, _tørrmelk_, kakaosmør, kakaomasse, emulgator (soyalecitin), aroma), palmeolje, sukker, hevemidler (ammoniumhydrogenkarbonat, natriumhydrogenkarbonat, dinatriumdifosfat), salt, aroma, fargestoff (betakaroten)- _Hvetemel_ -> en:wheat-flour - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 9410 - percent_min: 22.5 - percent_max: 77.5
- melkesjokolade -> en:milk-chocolate - vegan: no - vegetarian: yes - ciqual_food_code: 31004 - percent_min: 22.5 - percent: 22.5 - percent_max: 22.5
- sukker -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 3.75 - percent_max: 22.5
- _tørrmelk_ -> en:milk-powder - vegan: no - vegetarian: yes - ciqual_proxy_food_code: 19044 - percent_min: 0 - percent_max: 11.25
- kakaosmør -> en:cocoa-butter - vegan: yes - vegetarian: yes - ciqual_food_code: 16030 - percent_min: 0 - percent_max: 7.5
- kakaomasse -> en:cocoa-paste - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 16030 - percent_min: 0 - percent_max: 5.625
- emulgator -> en:emulsifier - percent_min: 0 - percent_max: 4.5
- soyalecitin -> en:soya-lecithin - vegan: yes - vegetarian: yes - ciqual_food_code: 42200 - percent_min: 0 - percent_max: 4.5
- aroma -> en:flavouring - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 3.75
- palmeolje -> en:palm-oil - vegan: yes - vegetarian: yes - from_palm_oil: yes - ciqual_food_code: 16129 - percent_min: 0 - percent_max: 22.5
- sukker -> en:sugar - vegan: yes - vegetarian: yes - ciqual_proxy_food_code: 31016 - percent_min: 0 - percent_max: 22.5
- hevemidler -> en:raising-agent - percent_min: 0 - percent_max: 18.3333333333333
- ammoniumhydrogenkarbonat -> en:e503ii - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 18.3333333333333
- natriumhydrogenkarbonat -> en:e500ii - vegan: yes - vegetarian: yes - percent_min: 0 - percent_max: 9.16666666666667
- dinatriumdifosfat -> nb:dinatriumdifosfat - percent_min: 0 - percent_max: 6.11111111111111
- salt -> en:salt - vegan: yes - vegetarian: yes - ciqual_food_code: 11058 - percent_min: 0 - percent_max: 1.24
- aroma -> en:flavouring - vegan: maybe - vegetarian: maybe - percent_min: 0 - percent_max: 1.24
- fargestoff -> en:colour - percent_min: 0 - percent_max: 1.24
- betakaroten -> en:e160ai - vegan: maybe - vegetarian: maybe - from_palm_oil: maybe - percent_min: 0 - percent_max: 1.24
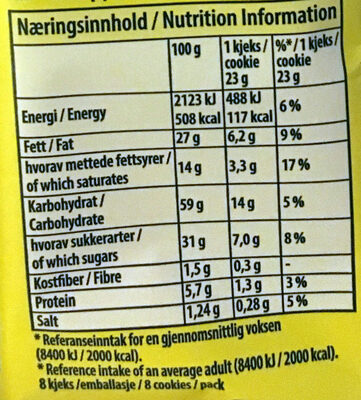
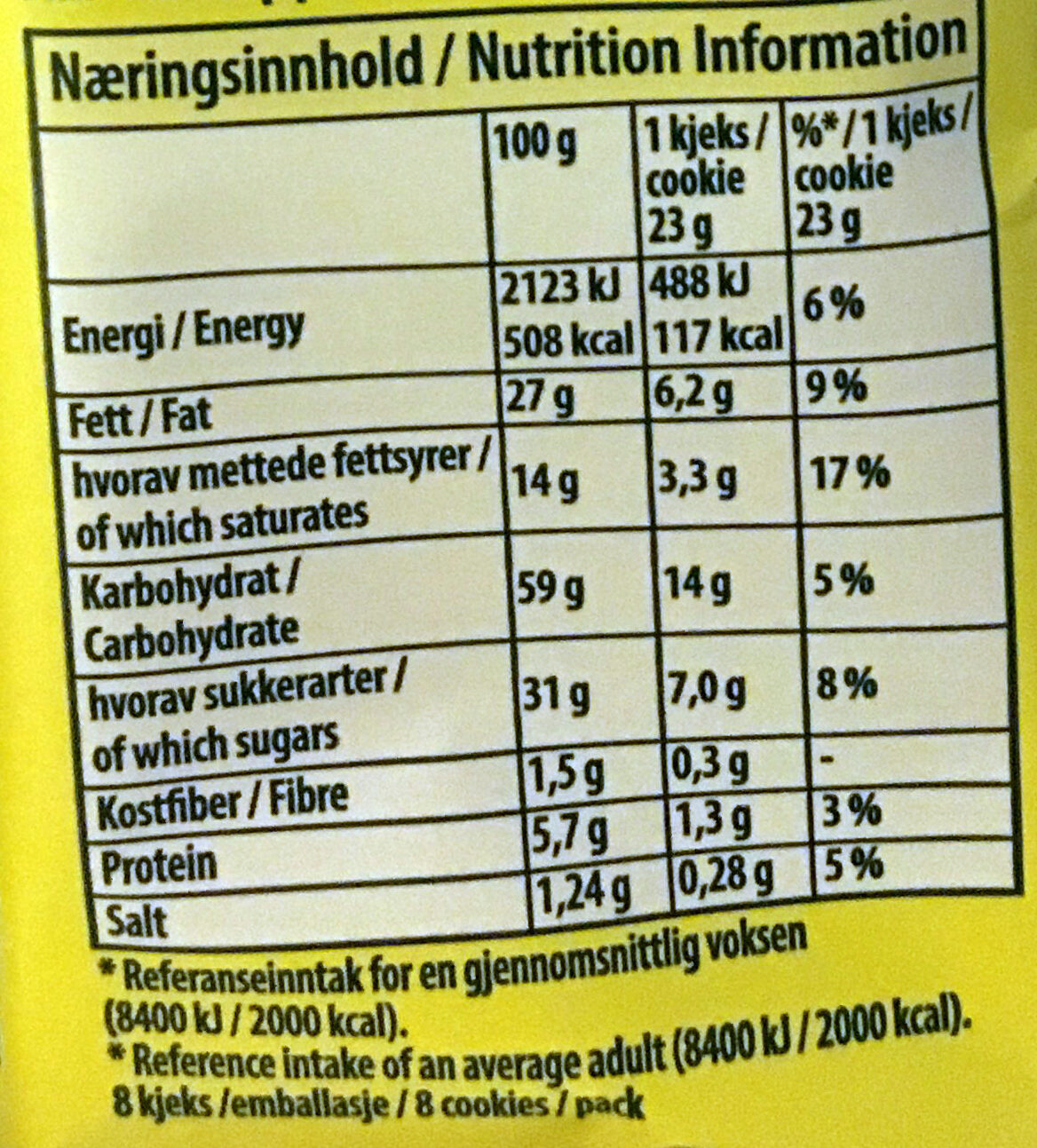
Ernæring
-
Dårlig ernæringskvalitet
⚠ ️Advarsel: mengden frukt, grønnsaker og nøtter er ikke spesifisert på etiketten, den ble anslått utfra ingredienslista: 0Dette produktet regnes ikke som en drikke under beregningen av Nutri-Score.
Positive poeng: 1
- Protein: 3 / 5 (verdi: 5.7, avrundet verdi: 5.7)
- Fiber: 1 / 5 (verdi: 1.5, avrundet verdi: 1.5)
- Frukt, grønnsaker, nøtter, og raps/valnøtt/olivenoljer: 0 / 5 (verdi: 0, avrundet verdi: 0)
Negative poeng: 27
- Energi: 6 / 10 (verdi: 2123, avrundet verdi: 2123)
- Sukker: 6 / 10 (verdi: 31, avrundet verdi: 31)
- Mettet fett: 10 / 10 (verdi: 14, avrundet verdi: 14)
- Natrium: 5 / 10 (verdi: 496, avrundet verdi: 496)
Poengene for proteiner telles ikke fordi de negative poengene er større eller lik 11.
Ernæringsverdi: (27 - 1)
Nutri-Score:
-
Næringsnivåer
-
Fett i høy kvantitet (27%)
Fint å vite- Et høyt inntak av fett, spesielt mettet fett, kan øke kolesterolet, som øker risikoen for hjertesjukdommer.
Anbefaling: Reduser inntaket av fett og mettet fett- Velg produkter med lavere fett- og mettet fettinnhold.
-
Mettet fett i høy kvantitet (14%)
Fint å vite- Et høyt inntak av fett, spesielt mettet fett, kan øke kolesterolet, som øker risikoen for hjertesjukdommer.
Anbefaling: Reduser inntaket av fett og mettet fett- Velg produkter med lavere fett- og mettet fettinnhold.
-
Sukkerarter i høy kvantitet (31%)
Fint å vite- Et høyt inntak av sukker kan føre til økning i vekt og tannråte. Det øker også risikoen for diabetes type 2 og hjerte- og karsjukdommer.
Anbefaling: Begrens inntaket av sukker og sukkerholdige drikker- Sukkerholdige drikker (som brus, fruktdrikker, og fruktjuicer og nektar) burde begrenses så mye som mulig (ikke mer enn 1 glass om dagen).
- Velg produkter med lavt sukkerinnhold og reduser inntaket av produkter med tilsatt sukker.
-
Salt i moderat mengde (1.24%)
Fint å vite- Et høyt inntak av salt (eller sodium) kan føre til et økt blodtrykk, som kan føre til høyere risiko for hjertesjukdom og slag.
- Mange folk har høyt blodtrykk uten å vite det, siden det ofte ikke er noen symptomer.
- De fleste inntar for mye salt (i gjennomsnitt 9 til 12 gram per dag), cirka det dobbelte av den anbefalte høyeste mengden av inntak.
Anbefaling: Begrens inntaket av salt og saltet mat- Reduser mengden salt som brukes ved matlaging, og ikke salt igjen ved bordet.
- Begrens inntaket av salte snacks og velg produkter med lavere saltinnhold.
-
-
Ernæringsinnhold
Ernæringsinnhold Som solgt
for 100 g / 100 mlSom solgt
per porsjon (23g)Sammenlignet med: en:Drop cookies Energi 2 123 kj
(508 kcal)488 kj
(117 kcal)+4 % Fett 27 g 6,21 g +12 % Mettet fett 14 g 3,22 g +23 % Karbohydrat 59 g 13,6 g −2 % Sukkerarter 31 g 7,13 g −11 % Kostfiber 1,5 g 0,345 g −36 % Protein 5,7 g 1,31 g +4 % Salt 1,24 g 0,285 g +68 % Fruits‚ vegetables‚ nuts and rapeseed‚ walnut and olive oils (estimate from ingredients list analysis) 0 % 0 %
Miljø
-
Eco-Score C - Moderat miljøavtrykk
Eco-Scoren er en eksperimentell poengsum som oppsummerer miljøavtrykket til matprodukter.→ Eco-Scoren ble opprinnelig utviklet for Frankrike og den blir nå utvidet til andre europeiske land. Eco-Score-formelen er i stadig endring og forbedres jevnlig for å gjøre den mer presis og bedre egnet til hvert land.Livssyklusanalyse
-
Gjennomsnittlig avtrykk for produkter i samme kategori: B (Score: 65/100)
Kategori: Biscuit (cookie), with chocolate drops
Kategori: Biscuit (cookie), with chocolate drops
- PEF-miljøscore: 0.38 (jo lavere poengsum, jo lavere avtrykk)
- inkludert innvirkning på klimaendringer: 4.22 kg CO2-ekv./kg produkt
Trinn Påvirkning Landbruk
49.8 %Bearbeiding
41.8 %Emballasje
2.9 %Transport
4.3 %Distribusjon
1.3 %Forbruk
0.0 %
Fordeler og ulemper
-
Mangler ingrediensopprinnelsesinformasjon
Ulempe: -5
⚠ ️ Opprinnelsen til ingrediensene til produktet er ikke angitt.
Hvis det er indikert på emballasjen, kan du redigere produktsiden og legge det til.
Hvis du er produsenten av produktet, kan du sende oss informasjon gjennom vår gratis plattform for produsenter.
-
Ingredienser som truer arter
Ulempe: -10
Inneholder palmeolje
Tropiske skoger i Asia, Afrika og Latin-Amerika ødelegges for å lage og utvide plantasjoner for palmeoljeproduksjon. Avskogingen bidrar til klimaendringer, og truer arter som orangutangen, dvergflodhesten og sumatraneshornet.
-
Mangler emballasjeinformasjon for dette produktet
Ulempe: -15
⚠ ️ Informasjonen om emballasjen til dette produktet er ikke fylt ut.⚠ ️ For en mer presis beregning av Eco-Scoren, kan du modifisere produktsiden og legge dem til.
Hvis du er produsenten av produktet, kan du sende oss informasjon gjennom vår gratis plattform for produsenter.
Eco-Score for dette produktet
-
Avtrykk for dette produktet: C (Score: 45/100)
Produkt: Melkesjokoladekjeks - Freia - 184g
Livssyklusanalysescore: 65
Sum av fordeler og ulemper: -20
Endelig poengsum: 45/100
-
Carbon footprint
-
Likt som å kjøre 2.2 km i en bensinbil
422 g CO² per 100g produkt
Karbonutslippstallet kommer fra ADEME's Agribalyse database, for kategorien: Biscuit (cookie), with chocolate drops (Kilde: Ademe Agribalyse Database)
Trinn Påvirkning Landbruk
27.9 %Bearbeiding
64.6 %Emballasje
2.6 %Transport
4.4 %Distribusjon
0.5 %Forbruk
0.0 %
Emballasje
-
Mangler emballasjeinformasjon for dette produktet
⚠ ️ Informasjonen om emballasjen til dette produktet er ikke fylt ut.Ta et bilde av resirkuleringsinformasjonen Ta et bilde av resirkuleringsinformasjonen
Transport
-
Opprinnelsen til ingredienser
Mangler ingrediensopprinnelsesinformasjon
⚠ ️ Opprinnelsen til ingrediensene til produktet er ikke angitt.
Hvis det er indikert på emballasjen, kan du redigere produktsiden og legge det til.
Hvis du er produsenten av produktet, kan du sende oss informasjon gjennom vår gratis plattform for produsenter.Legg til opprinnelsen til ingrediensene i dette produktet Legg til opprinnelsen til ingrediensene i dette produktet
Threatened species
-
Inneholder palmeolje
Fører til avskoging og truer arter som orangutangen
Tropiske skoger i Asia, Afrika og Latin-Amerika ødelegges for å lage og utvide plantasjoner for palmeoljeproduksjon. Avskogingen bidrar til klimaendringer, og truer arter som orangutangen, dvergflodhesten og sumatraneshornet.
Etiketter
Report a problem
-
Incomplete or incorrect information?
Category, labels, ingredients, allergens, nutritional information, photos etc.
If the information does not match the information on the packaging, please complete or correct it. Open Food Facts is a collaborative database, and every contribution is useful for all.
Datakilder
Produkt lagt til av kiliweb
Siste redigering av produktsiden den av spotter.
Produktside også redigert av odinh, openfoodfacts-contributors, yogoff, yuka.R1BwUkxMeGRwcU5Rc3ZGajVoTHMrdEZlOXFPcFdqNjdkdFpBSUE9PQ.